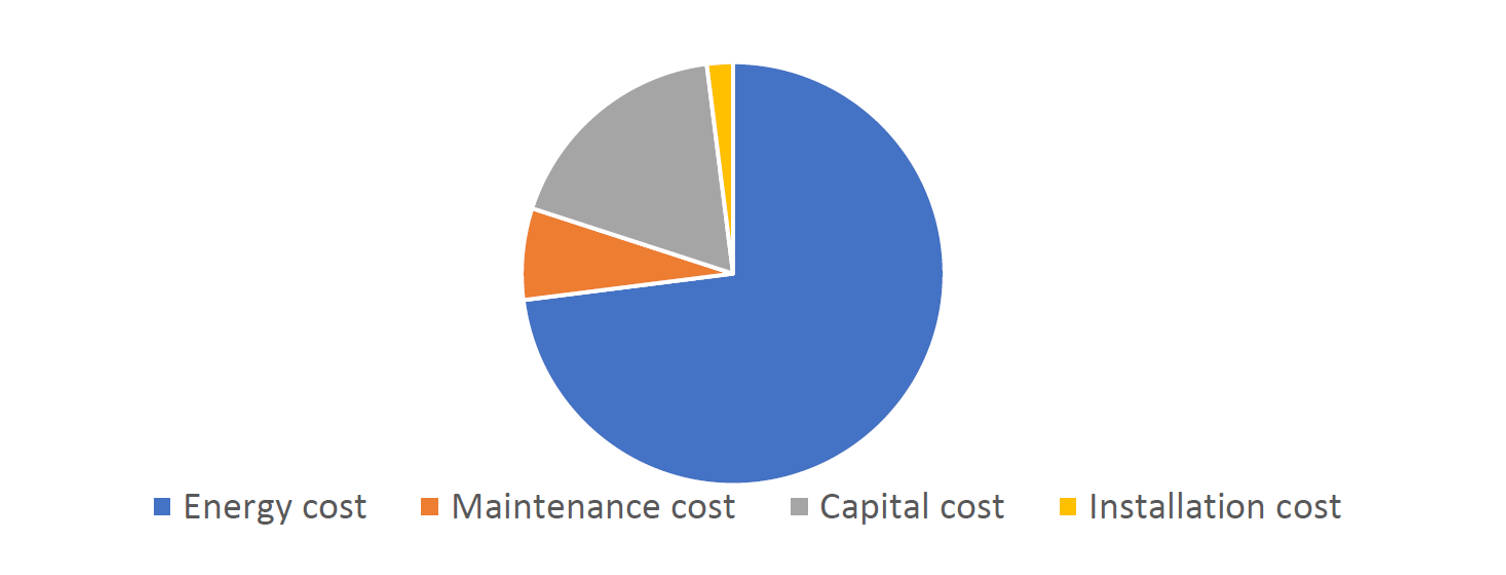
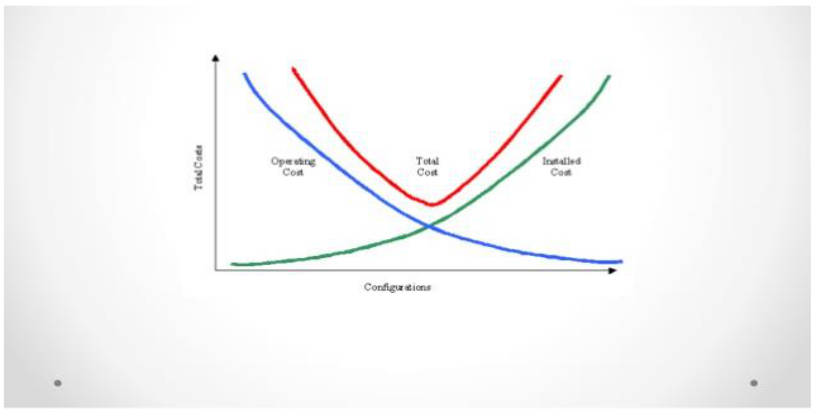
Pharma manufacturers today operate in one of the most demanding business environments. Every strategic decision is shaped by two critical performance indicators: Cost Per Thousand units (CPT) and Overall Equipment Effectiveness (OEE). These ultimately define profitability and operational efficiency in modern pharma facility design.
The challenge is that the market does not wait. It expects higher quality, lower costs, and faster delivery, all while avoiding excess inventory. Demand patterns swing drastically. A product may require a very small batch one month and massive volumes the next.
This unpredictability creates a dilemma. Adding more equipment may seem like an easy solution, but it lowers OEE and increases depreciation directly impacting profitability. On the other hand, under-preparedness risks delays, compliance pressure, and lost market opportunities.
This is why pharma manufacturing facilities must evolve. They need to be lean enough to minimize waste and capital burden, yet flexible enough to adapt to demand shifts without compromising quality- a core principle of lean pharma manufacturing.
To overcome these challenges, modern pharma facilities should be designed with the following four aspects in mind:
- Building Facility Lean
- Equipment Selection with Flexibility
- Single-use Systems
- Automation and Industry 4.0
1. Building a Lean Pharma Facility
Quality is simply conformance to requirements. A lean pharma facility must be compact, focused, and designed with both capital investment and operating costs in mind. This is the foundation of effective pharma turnkey solutions.
Facilities should be planned with at least 10 years of visibility, as regulatory requirements, customer expectations, and processing technologies evolve rapidly. Without this foresight, organizations risk costly revamps far sooner than anticipated.
Key principles of lean facility design include:
- Keeping facilities compact and requirement-driven to control both capital expenditure and operating expenses
- Focusing on core manufacturing activities while outsourcing non-core functions such as warehousing, pharma engineering services, and selected quality activities to reduce total cost of ownership (TCO)
- Placing only essential equipment inside cleanrooms and shifting support equipment to service areas to minimize cleanroom footprint and operating costs
- Challenging design tolerances wherever possible reducing unnecessary overengineering (for example, tighter tolerances beyond ±2%) directly lowers capital and lifecycle costs
A lean facility design reduces depreciation impact, improves OEE, and helps manufacturers keep CPT competitive in a dynamic and unpredictable market.

2. Flexible Equipment Selection for Variable Batch Sizes
Variation in batch size is one of the biggest operational challenges in pharmaceutical manufacturing. Very small production runs and large-scale volumes cannot be efficiently addressed by simply adding more equipment, as this approach reduces OEE and increases depreciation.
Instead, manufacturers should focus on flexible equipment strategies, including:
- Selecting equipment capable of efficiently handling both small and large batch sizes
- Prioritizing shorter changeover times to improve operational efficiency without compromising quality or compliance
- Investing only in essential options initially, while keeping the ability to scale or upgrade as product and market needs evolve
- Evaluating equipment not just on output capacity and cost, but also on flexibility, reliability, and quality performance
Flexible equipment enables pharma manufacturers to remain agile, respond to demand fluctuations, and align capital investment with actual business needs.
3. Single-Use Systems in Modern Pharma Facilities
Single-use systems have transformed how pharmaceutical facilities are designed and operated, especially in environments where product changeovers, batch variability, and contamination control are critical.
Traditional stainless-steel systems demand extensive cleaning, validation, and downtime. In contrast, single-use technologies significantly reduce these burdens while improving operational flexibility.
Key advantages of single-use systems include:
- Eliminating cleaning-in-place (CIP) and sterilization-in-place (SIP) requirements, resulting in faster changeovers and higher equipment availability
- Reducing cross-contamination risks, which enhances product quality and regulatory confidence
- Enabling rapid scale-up or scale-down without major capital investment
- Lowering water, energy, and utility consumption, supporting both cost reduction and sustainability goals
Single-use systems are particularly effective for multi-product facilities, clinical manufacturing, and operations with highly variable demand. When applied strategically, they help manufacturers improve OEE while keeping capital expenditure aligned with real production needs.
4. Automation and Industry 4.0 in Pharma Manufacturing
Automation and Industry 4.0 are no longer optional upgrades they are foundational elements of future-ready pharma facilities. When implemented correctly, automation improves consistency, compliance, and operational visibility across the manufacturing lifecycle.
Modern automation strategies go beyond basic control systems. They integrate data, equipment, and people to enable smarter decision-making through pharma automation solutions.
Core benefits of automation and Industry 4.0 include:
- Reducing manual interventions, thereby minimizing human error and improving batch consistency
- Enabling real-time monitoring of critical process parameters, equipment performance, and quality attributes
- Improving OEE through predictive maintenance and data-driven performance optimization
- Strengthening data integrity and compliance with regulatory expectations such as ALCOA+ principles
A key consideration is scalability. Automation systems should be designed in modular layers, allowing facilities to start with essential controls and expand toward advanced analytics, digital twins, and artificial intelligence as maturity increases.
When aligned with lean facility design and flexible equipment strategies, automation becomes a powerful enabler of efficiency rather than an added cost burden.
Where Pharma Access Fits In
At Pharma Access, we help manufacturers design and build pharma facilities that are lean, flexible, and future-ready. From smart equipment selection and modular facility concepts to Industry 4.0 enabled solutions, we support our clients in reducing costs, improving OEE, and maintaining long-term regulatory compliance.
In today’s pharmaceutical industry, success is not about building bigger facilities it is about building smarter, faster, and more adaptable operations.
And that is exactly what we deliver.