When Infrastructure Becomes the Bottleneck
Pharmaceutical innovation is moving so fast that, in some cases, products are approved before the facilities meant to manufacture them are ready. In many cases, traditional pharma facilities are still designed for longer development cycles and predictable demand, making it increasingly difficult for infrastructure to keep pace with Modular Pharmaceutical Facilities.
In several recent cases, companies have invested hundreds of millions of dollars in new plants, only to face delayed integration, extended qualification timelines, or underutilized capacity when market assumptions changed. What should have been a moment of commercial momentum instead became a race against infrastructure.
This is not a failure of science. It is a mismatch between how quickly innovation now moves and how slowly manufacturing capacity is still brought online.
The pharmaceutical industry has entered an era defined by rapid modality shifts, compressed development timelines, and uncertain long-term demand. Yet most drug manufacturing facilities are still designed for a world where demand was predictable, portfolios changed slowly, and long-term utilization could be assumed.
That gap is becoming increasingly difficult and increasingly costly to ignore.
Why Traditional Facility Delivery Is Struggling to Keep Pace
Traditional pharmaceutical manufacturing facilities are delivered through long, sequential processes, with engineering, construction, integration, and qualification largely occurring in series. This delivery model leaves limited room to absorb change once projects are underway- particularly when disciplines such as engineering design, construction and installation, and CQV are not fully aligned early in execution.
Industry bodies acknowledge the resulting pressure. As ISPE has noted, “many projects nowadays are required to be delivered in very short and challenging timescales, increasing the risk of design and delivery errors that may compromise project and facility requirements and operation.” Late-stage design clarification and compliance alignment can therefore extend timelines disproportionately during integration and qualification.
At the same time, traditional facilities struggle to adapt as requirements evolve. BioPhorum observes that “traditional biomanufacturing facility construction projects can be expensive to build and modify and may lack flexibility to accommodate new products.” In response, the organization argues that “moving towards more standardized, modular design and construction solutions is needed to meet the demands of the biopharmaceutical market.”
Together, these observations point to a structural limitation. Conventional facility delivery assumes stability at a point when manufacturing requirements are still changing, making speed, flexibility, and predictability increasingly difficult to achieve.
Rethinking Where and How Pharmaceutical Facilities Are Built
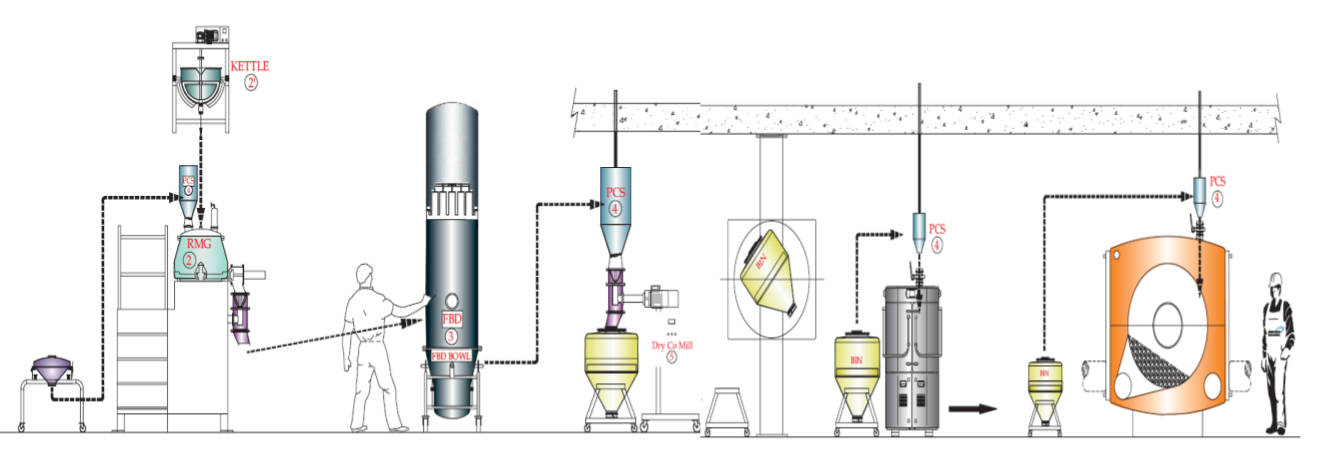
In response to these constraints, some organizations are beginning to rethink not just how pharmaceutical facilities are built, but where critical work is performed. Rather than executing the entire project on-site, modular and prefabricated approaches shift significant portions of fabrication, integration, and testing into controlled factory environments.
This change alters the structure of delivery. Workstreams that traditionally occur in sequence can progress in parallel, reducing dependence on site conditions and local labour availability. Equipment skids, cleanroom modules, electrical and piping systems and utility systems can be assembled and tested off-site, allowing quality activities to begin earlier and reducing the volume of late-stage rework during qualification.

Industry groups such as ISPE and BioPhorum have increasingly highlighted modular construction as a practical response to schedule pressure and design volatility. The appeal is not limited to speed alone. Off-site fabrication offers greater repeatability, improved quality control, and earlier visibility into integration challenges.
Importantly, modular approaches are not positioned as a universal replacement for traditional facilities. Instead, they represent an emerging option for organizations seeking to balance compliance requirements with faster deployment and greater adaptability in uncertain environments.
Why Partial Modular Adoption Falls Short
While modular and prefabricated elements are gaining traction, many organizations initially adopt them as add-ons to traditional delivery models. Skids, prefabricated rooms, or utility modules are inserted into otherwise sequential projects, with limited impact on overall timelines or risk profiles.
This partial adoption often underdelivers. When modular components are treated as isolated efficiencies rather than part of a broader delivery strategy, critical constraints remain unchanged. Design decisions are still locked in early, integration is still deferred to the site, and qualification remains concentrated at the end of the project – a dynamic explored further in discussions on risk management in pharmaceutical project execution.
The real value of modular approaches emerges only when they are used to restructure how work is sequenced and where risk is absorbed. Off-site integration, parallel execution, and earlier testing fundamentally change the delivery dynamic, reducing late-stage congestion and increasing predictability.
This distinction matters. Modular construction is not simply about building faster. It is about changing how uncertainty is managed within the facility delivery process.
The Business Case for Modular and Prefabricated Facilities

Industry research reinforces why modular and prefabricated approaches are gaining traction across pharmaceutical manufacturing. Market studies focused specifically on pharma and biotechnology facilities, including analyses by ResearchAndMarkets and Roots Analysis, indicate that modular pharmaceutical plants can be delivered up to 40 percent faster than conventional stick-built facilities, significantly shortening time-to-capacity.
The same studies associate modular construction with 25 to 30 percent reductions in overall construction costs, driven by parallel execution, off-site fabrication, and reduced site-level disruption. Additional market analysis from organizations such as GlobalInfoResearch supports these findings, noting that modular approaches improve schedule predictability and reduce delivery risk compared to traditional construction models.
Together, these findings suggest that modular prefabrication is no longer a niche alternative, but an increasingly validated approach for organizations seeking faster deployment, improved predictability, and more disciplined capital use in pharmaceutical manufacturing – aligning closely with principles outlined in Smarter Pharma Facilities: Lean, Flexible & Future-Ready.
Where Modular Approaches Deliver the Greatest Value
Modular and prefabricated approaches create the greatest impact in manufacturing environments where speed, capital discipline, and sustainability objectives intersect. Their value becomes particularly clear when the benefits are viewed across execution, cost, and long-term flexibility.
Key benefits in practice include:
- Faster deployment:
By shifting fabrication and integration into controlled factory environments and enabling parallel execution, modular delivery significantly shortens time-to-capacity and reduces reliance on site conditions. - Improved capital efficiency:
Standardized designs and off-site fabrication reduce rework, limit site disruption, and lower overall delivery costs -reinforcing lifecycle-focused thinking discussed in Lifecycle Costing & Capital Budgeting. - Greater predictability during qualification:
Earlier integration and factory-based testing reduce late-stage congestion and improve first-time-right outcomes in GMP environments. - Enhanced sustainability performance:
Modular execution typically reduces material waste, lowers concrete usage, and shortens on-site construction activity, contributing to a lower construction-phase environmental footprint. - Built-in expandability:
Repeatable layouts and pre-engineered systems enable faster future expansions with less disruption to ongoing operations.
To put these benefits into practical context, consider a 15,000 sqm pharmaceutical manufacturing facility executed using a modular prefabricated (MMF) approach and compared against a conventional site-built delivery on a like-for-like basis:
- ~30% faster completion, driven by parallel workstreams and reduced site dependency
- ~25–30% lower delivery costs, reflecting standardization and reduced rework
- Substantially lower concrete usage and a material reduction in construction-phase carbon footprint
- ~25% faster future capacity expansion due to modular layouts and pre-engineered systems
While outcomes vary by project and context, this comparison highlights how modular prefabrication can materially improve speed, cost efficiency, and sustainability relative to traditional execution models.
Designing Infrastructure for an Uncertain Future
The pharmaceutical industry has always balanced innovation with regulation and long-term investment. What has changed is the pace at which those balances must now be struck. Manufacturing infrastructure decisions made today shape not only operational performance, but strategic flexibility for years to come.
Traditional facilities will remain essential for large-scale, long-term production. However, the analysis presented here shows that they are no longer the only answer. Modular and prefabricated approaches offer a complementary way to deploy capacity faster, manage capital risk more deliberately, and reduce environmental impact, particularly in environments where demand, technology, or market access remain uncertain.
For industry leaders, the question is no longer whether modular approaches are technically viable. It is whether infrastructure strategies are sufficiently aligned with portfolio realities and risk tolerance. Manufacturing infrastructure can no longer be treated as a static asset designed solely for scale and longevity. In today’s operating environment, facilities increasingly determine how quickly scientific progress can be translated into commercial reality – a theme also explored in What It Takes to Build Pharmaceutical Facilities from Day One.

For organizations planning new or expanded manufacturing capacity, the following steps can help align infrastructure decisions with current operating realities:
- Reassess infrastructure assumptions early, before design and capital commitments become irreversible.
- Evaluate modular and prefabricated options alongside traditional builds, not as afterthoughts.
- Model infrastructure decisions at the portfolio level, considering speed, flexibility, and sustainability in addition to capacity.
- Engage regulatory and quality teams early to align qualification strategies with evolving delivery models.
In practice, modular and mobile facility platforms are beginning to translate these principles into deployable infrastructure. Modular Mobile Facility (MMF) offers an example of how standardized, prefabricated, and turnkey facility concepts can be applied to pharmaceutical manufacturing. Built around pre-engineered and pre-validated design platforms, MMF’s approach illustrates how modular strategies can support faster deployment, reduced execution risk, and scalable expansion in regulated environments.
In an industry defined by uncertainty, the competitive advantage may no longer lie in building the largest or most permanent facilities. It lies in designing infrastructure that can be deployed predictably, adapted over time, and aligned with the realities of innovation itself.