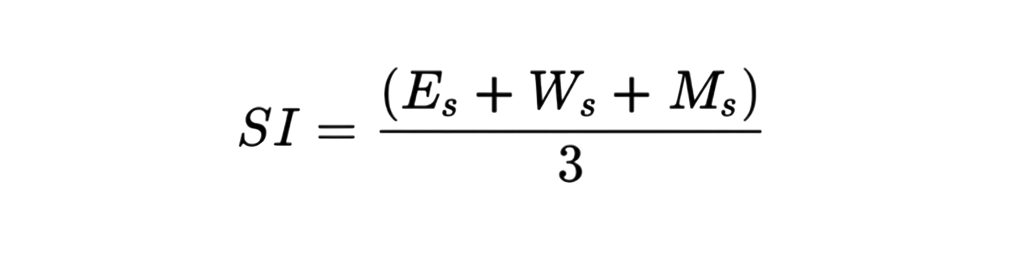Why Skipping Quality Costs More Than You Think
In today’s hyper-competitive industrial landscape, delivering projects on time and within budget isn’t just a goal—it’s a mandate. Yet, countless projects continue to bleed time and money due to a single, often underestimated culprit: poor quality.
It’s a myth that quality is expensive. The truth? Not investing in quality is far costlier.
What is Quality?
Quality in engineering or project execution refers to the ability to meet predefined functional, regulatory, and client expectations consistently. It is not limited to aesthetics or minimal compliance; instead, it represents reliability, fitness for purpose, and error-free execution across project delivery.
“Access is not quality. Less is not cost-effective.”
Quality is about balance. It ensures that all stakeholders such as client, consultants, vendors, and contractors are aligned on expectations and delivery benchmarks.
Understanding the Cost of Poor Quality (CoPQ)
The CoPQ includes all costs incurred due to work not being done correctly the first time. These costs can be broken down into:
- Rework
- Wastage (materials, manpower, time)
- Penalties due to non-compliance
- Delays in project completion
- Equipment damage or underperformance
- Idle time due to mismatched sequencing
Studies indicate that on average, 5%-10% of a project budget is lost to poor quality.
In large-scale pharmaceutical projects, this translates to tens of lakhs or more. That’s not just a figure on a spreadsheet. That’s budget that could have funded expansion, innovation, or simply been added to the bottom line.
How to Calculate CoPQ?
To quantify the Cost of Poor Quality (CoPQ), we use an internally developed scoring model that evaluates each identified issue based on three weighted parameters:
Each identified issue is evaluated using the following parameters:
Impact (I): Severity of effect on the project (1–5 scale)
Frequency (F): Rate of occurrence (1–5 scale)
Control (C): Ease of prevention (1–5 scale)
Formula: CoPQ Score = I × F × C
In this model, higher scores for Impact and Frequency indicate more damaging and frequent issues, while a lower Control score reflects a greater lack of control, making the issue harder to prevent.
Decoding the True Cost of Poor Quality in Pharma
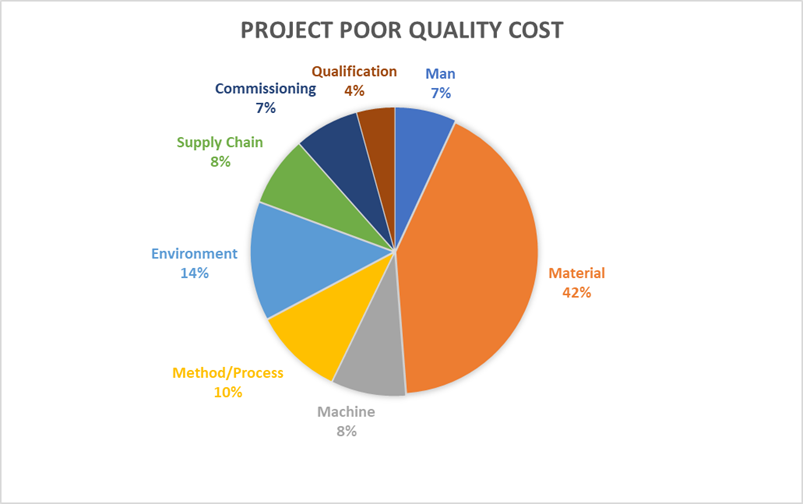
Quality failures in pharmaceutical manufacturing are rarely the result of a single misstep. More often, they’re a cumulative effect — a breakdown across systems, processes, and materials. One of the diagrams we examined offers a clear visual of this: poor quality doesn’t just show up as a defect; it shows up as rework, complaints, scrap, and even product recalls.
Let’s unpack this further through a data-driven lens
1. Material: The Dominant Cost Contributor (42%)
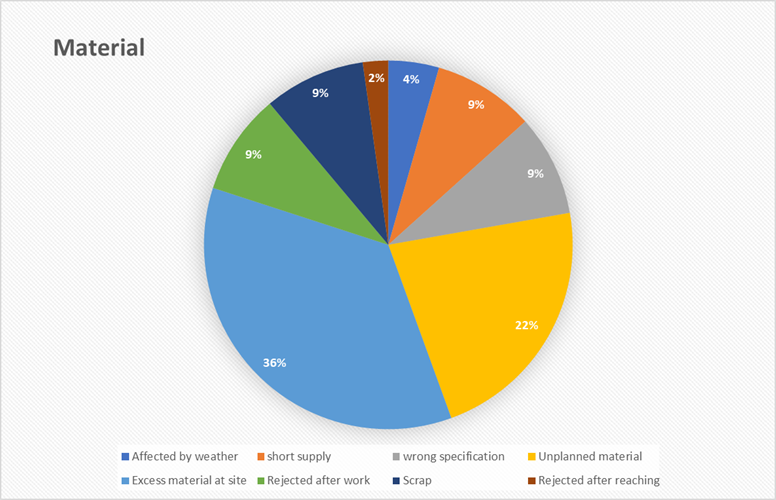
Effective material management is the logistical backbone of any successful project. Failures in this area create foundational cracks that ripple outwards, causing budget overruns, on-site clutter, and critical path delays long before the first component is ever installed.
Key Takeaways:
- Implement dynamic inventory controls to prevent costly over-ordering and stockouts.
- Strengthen vendor qualification and compliance checks to ensure material quality meets specifications.
- Improve demand forecasting to minimize reliance on last-minute, unplanned material requisitions.
2. Method: The Operational DNA (10%)
A project’s methodology is its operational DNA. It dictates the standard of work, safety, and efficiency. When this DNA is flawed through poor design, lack of standards, or unsafe shortcuts, it leads to systemic, not isolated, failures that compromise the integrity of the entire project.
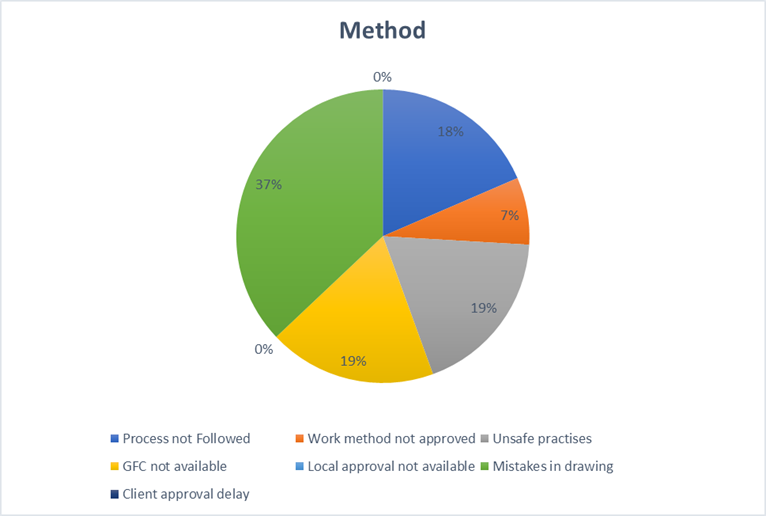
Key Takeaways:
- Mandate a multi-stage design and drawing validation process to catch critical errors early.
- Enforce strict adherence to safety protocols and the use of approved, construction-ready drawings.
- Standardize key workflows and conduct regular compliance audits to ensure processes are followed.
3. Manpower: Human Factors Matter (7%)
A project’s success ultimately depends on its people. Success requires more than just filling roles; it demands having the right individuals who are properly equipped, physically ready, and clearly aligned with their tasks. Gaps in workforce readiness create immediate and significant friction.
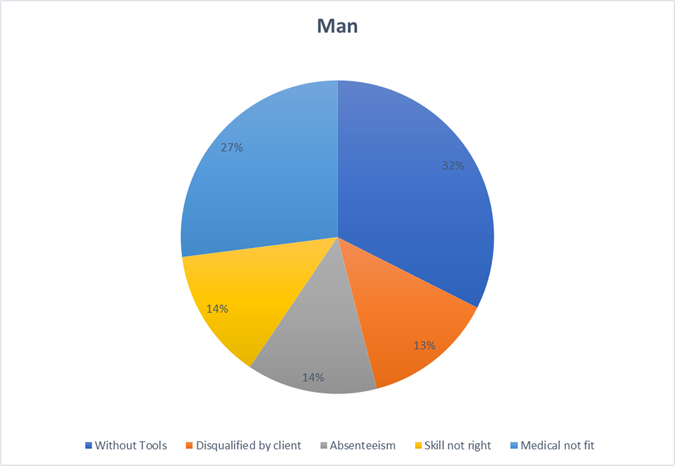
Key Takeaways:
- Establish a “ready-to-work” protocol to ensure all personnel are properly equipped before deployment.
- Integrate workforce well-being and fitness-for-duty checks into the resource planning process.
- Align skills to specific tasks through more effective vetting and role-based training.
4. Machine: Technology & Equipment Gaps (8%)
Technology and equipment are meant to be project accelerators. However, when mismanaged, they become expensive anchors, dragging down progress due to unavailability, non-compliance, or poor maintenance. Strategic asset management is crucial for keeping the project moving forward.
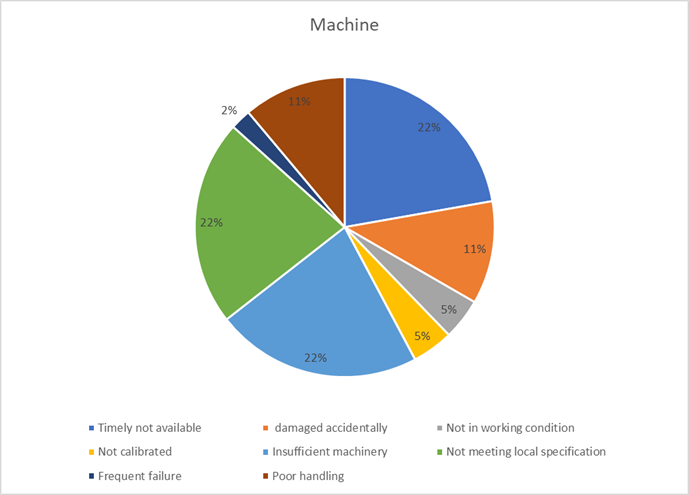
Key Takeaways:
- Develop a strategic procurement plan that ensures the timely availability of compliant machinery.
- Conduct thorough needs assessments to prevent the over- or under-provisioning of critical equipment.
- Implement a proactive maintenance and calibration schedule to maximize uptime and reliability.
5. Environment: Contextual Challenges (14%)
While external conditions like weather are often uncontrollable, a project’s response to them is not. Proactive planning transforms an organization from a victim of its environment into a resilient entity capable of navigating unforeseen challenges with minimal disruption.
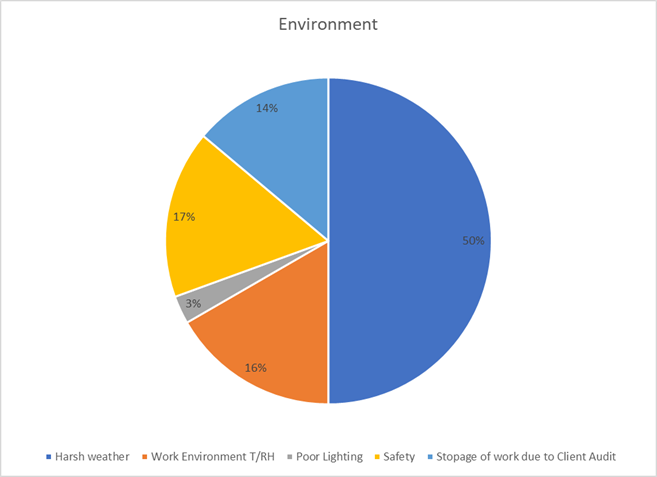
Key Takeaways:
- Integrate weather-related contingency plans and buffer times into all project schedules.
- Develop clear protocols for managing work stoppages from audits or safety events.
- Prioritize site safety, including proper lighting, to mitigate environmental hazards.
6. Supply Chain: Delays and Disruptions (8%)
The supply chain is a sequence of promises. When a single promise is broken whether by a logistics provider or a parts supplier, the delay creates a ripple effect that can disrupt the entire project schedule. A resilient supply chain is a non-negotiable asset.
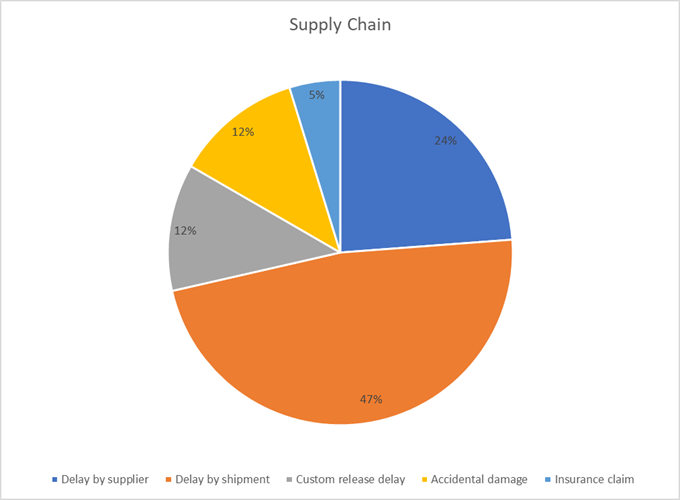
Key Takeaways:
- Optimize shipping routes and diversify logistics partners to reduce critical transit delays.
- Strengthen supplier SLAs with clear performance metrics and penalties for non-performance.
- Digitize documentation to streamline customs clearance and prevent administrative hold-ups.
7. Commissioning & Qualification: Final Mile Misfires (11%)
The commissioning and qualification phase are the project’s moment of truth. Failures at this late stage are highly visible and disproportionately costly, often revealing a lack of integrated planning, poor communication, and weak discipline from the preceding months.
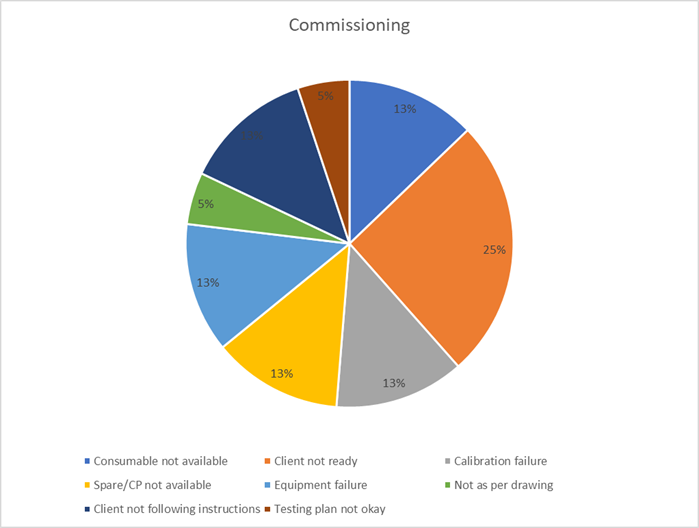
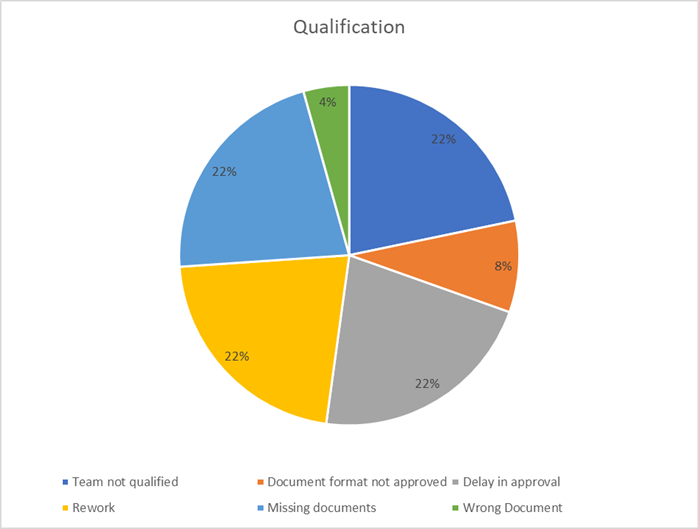
Key Takeaways:
- Ensure client and stakeholder readiness through joint planning and transparent communication.
- Maintain rigorous documentation and team qualification standards from day one, not just at the end.
- Pre-plan for final-stage logistics, ensuring all consumables, spares, and tools are on hand.
The data is clear: prevention pays. A facility or organization that prioritizes design validation, supplier integrity, and in-line process control doesn’t just make better products, it spends less fixing problems and builds greater trust in the market.
It’s time to rethink how and where we invest in quality.
The Real Cost of Inefficiency: An ₹8 Crore Story
A line chart tracking planned vs. actual costs across months revealed a clear trend: projects consistently overshot budgets due to recurring quality-related issues.
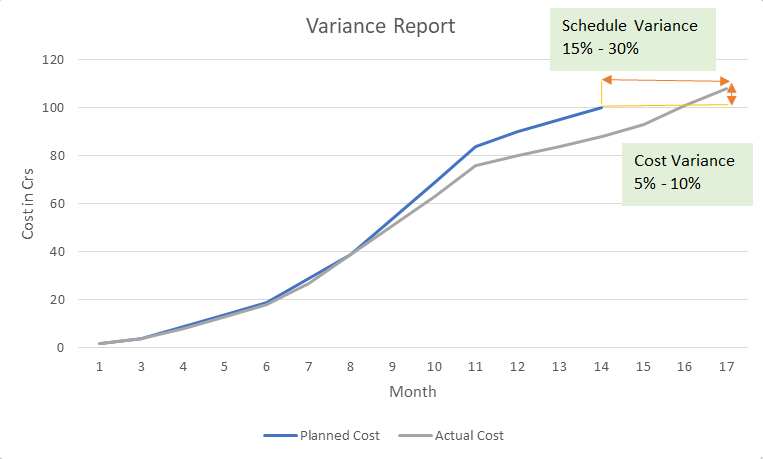
These weren’t one-off errors. They were systemic issues that repeated due to the lack of a quality-first mindset. The issues detailed above are not theoretical. They have tangible financial consequences. Consider a real-world project scenario:
- The Plan: Deliver a project in 14 months with a budget of ₹100 Crores.
- The Reality: The project was completed in 17 months at a final cost of ₹108 Crores.
This isn’t just a minor variance. It’s an ₹8 Crore loss and a three-month delay that eroded profits and damaged client trust. Where did that money go? It was consumed by the very issues we’ve analyzed:
- A portion was lost to Material (42% = ₹ 3.36 Cr) when excess materials were ordered and stored, while work stalled waiting for the correct materials to arrive after a quality rejection.
- Productivity plummeted due to Manpower (7% = ₹0.56 Cr) issues, with improperly equipped teams unable to perform their tasks, causing cascading delays.
- More was wasted on rework caused by Method (7% = ₹0.56 Cr), where teams built according to flawed drawings that had to be corrected mid-stream.
- Machine-related delays (8% = ₹0.64 Cr) also took their toll, whether through late equipment deliveries, mismatched installation readiness, or on-site adjustments that pushed timelines and resources out of sync.
- Neglecting early precautions meant Environmental factors (14% = ₹1.12 Cr) kicked in like water damage, dust contamination, or poor sealing forcing corrective action that could’ve been avoided with upfront discipline.
- The timeline was extended by Supply Chain (8% = 0.64 Cr) failures, as critical components sat idle in transit, pushing back the entire project schedule.
- Finally, the project stumbled across Commissioning & Qualification (11% = ₹0.88 Cr), with commissioning delayed because the client wasn’t ready and key documents were missing, adding weeks of overhead costs at the very end.
All of it could have been avoided with a modest investment in quality planning, vendor audits, skilled labor onboarding, and document validation. In most cases, that investment wouldn’t have exceeded ₹50–60 lakhs—less than 1% of the total project cost. Instead of bleeding ₹8 Crores across invisible cracks, the project could have gained speed, saved money, and earned client trust.
It’s a clear case: proactive discipline always costs less than reactive damage control.
Flip the Script: Quality as a Strategic Lever
Investing in quality isn’t about checklists. It’s about risk mitigation. It’s about leadership. It’s about creating a culture where mistakes are prevented not corrected after the fact.
At the heart of this transformation is a simple idea:
“Start with quality. End with savings.”
This philosophy shifts quality from being a compliance requirement to a strategic differentiator. It’s how the best projects are delivered on time, on budget, and with lasting value.
Final Word
Project delays and quality issues are closely intertwined and can significantly impact project outcomes. Delays can lead to increased costs, missed deadlines, and reputational damage, while quality issues can result in rework, customer dissatisfaction, and safety hazards. Addressing these issues requires proactive planning, effective communication, and robust quality management processes.
In an era where margins are thin and expectations high, quality is the smartest investment you can make. It protects your timeline, preserves your reputation, and ultimately ensures that every rupee you spend is moving your project forward—not cleaning up mistakes from the past.
Let’s not treat quality as a cost.
Let’s treat it as the value engine it truly is.